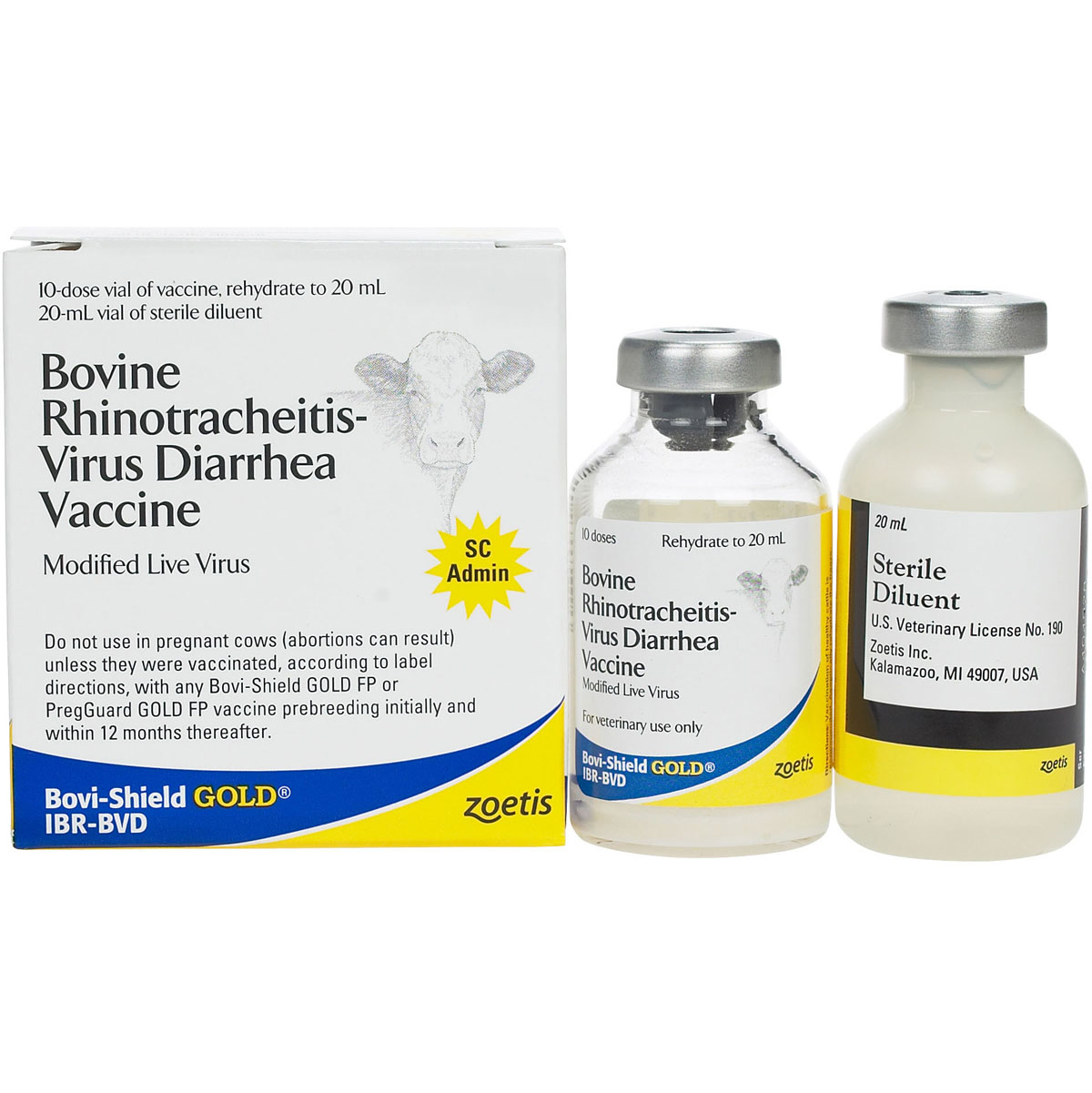
BOVI SHIELD GOLD IBR BVD 10 DS 20 ML/BT
Case Qty: 100
Vaccine, 20 mL Container, Bottle Container, 2 mL Dosage, 10 Dose, Bovine Rhinotracheitis, Virus Diarrhea, modified Live Virus, IBR, BVD Type 1 and 2 Kills, Viremia, Respiratory Diseases, Gentamicin Composition, AICS, EU EINECS/ELINCS Standards, Calves Vaccinated Before 6 Months of Age Should be Revaccinated at 6 Months, For Healthy Cattles
QUALIFIES FOR FREE FREIGHT ON ORDERS OVER $1000